Insights into neural stem cell research » BRAIN AND PATH » SciLogs
Doctors are regularly confronted with diseases whose cruelty is heartbreaking. Epidermolysis Bullosa is such a disease: the skin of the terminally ill reacts to even the slightest contact and stimuli with blistering and peeling, so that infections, scars and pain become a constant companion. Most of those affected die in childhood and adolescence. This made the report in the renowned New England Journal of Medicinethat for the first time a 7-year-old boy was cured of his disease (1). The cure: a retransplantation of his own stem cells.
Stem cell therapies are on the rise
The cause of epidermolysis bullosa lies in faulty genes that can no longer produce proteins in the skin. This is where stem cell therapy comes in. The young patient’s stem cells were removed and healthy genes were inserted into them (using the CRISPR-Cas method, known as ‘gene scissors’). The modified stem cells could then be re-transplanted into the patient. In the patient’s own body, they then produced healthy, stable skin cells – blisters, peeling and pain were a thing of the past, and the patient was cured.
This was not the first case in which stem cells were able to cure a difficult disease. In sickle cell anemia, stem cells, similar to epidermolysis bullosa, create tissue incorrectly. Only instead of skin cells, they are red blood cells (erythrocytes). These are formed in a sickle shape rather than an elliptical shape, so that they become tangled and clump together, disrupting blood circulation and causing heart attacks. As in the case of the boy with skin disease, a few years ago a patient was experimentally cured of her anemia at the University Hospital of Regensburg. Her own stem cells were also removed, modified and reinserted. The Regensburgers recently published a study (2) in which 29 out of 30 patients were cured in this way.
With regard to the effectiveness of stem cell therapies, countless patients are asking themselves the question: would neural stem cells also be possible as a therapy for diseases of the nervous system? Will we soon be able to cure Alzheimer’s or Parkinson’s?
What are stem cells?
In order to properly assess the potential and prospects of neural stem cell therapy, e.g. for Alzheimer’s or Parkinson’s, one must consider the function of stem cells in general and the peculiarities of neural tissue.
We define stem cells as cells that are not yet determined to a tissue type, meaning that they can still differentiate into a variety of special cells. But not all stem cells are the same. We differentiate between omnipotent or totipotent stem cells and pluripotent and multipotent stem cells. A complete organism can grow from an omnipotent or totipotent stem cell, an example would be the fertilized egg cell. Pluripotent stem cells can still differentiate into all tissue types. Multipotent stem cells, on the other hand, only differentiate into the specialized cells one tissue type such as skin or blood cells. A final relevant type are oligopotent stem cells, which can only differentiate into a few cell types of a tissue type. Most of the remaining cells are unipotent or post-mitotic, meaning they can only produce cells of their own type or are no longer able to divide at all.
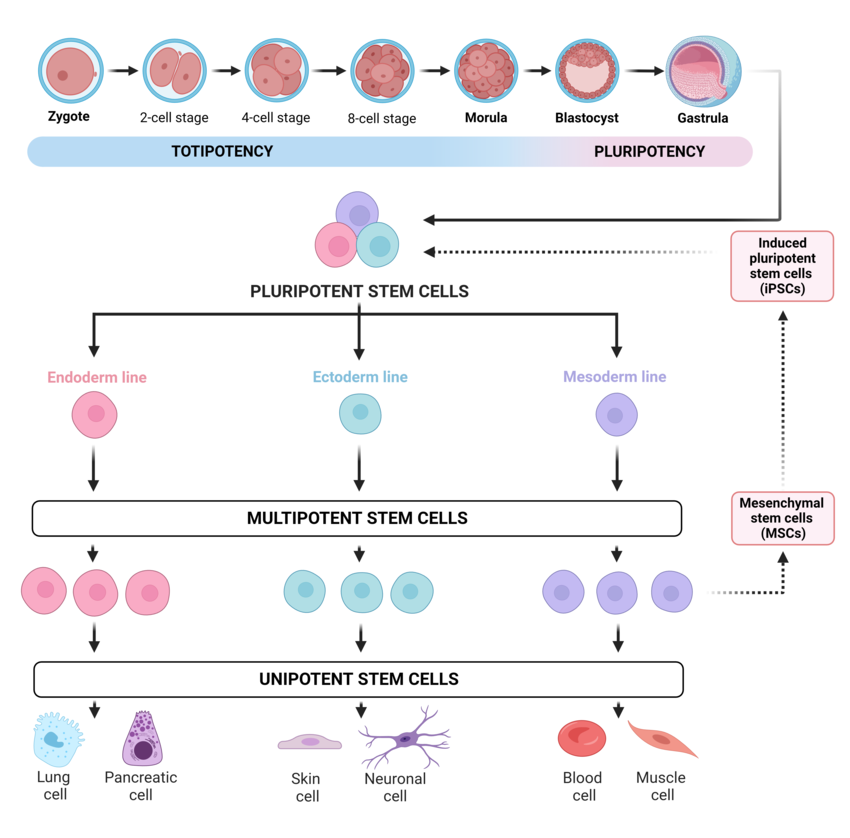
In most parts of the body and organs, even adults have deposits of adult, multipotent stem cells that ensure that tissue is reproduced there. They also play an important role in replacing lost tissue in the event of injuries. If you imagine neurodegenerative diseases such as Alzheimer’s as ‘brain injuries in slow motion’, then it is understandable that many patients ask whether neural stem cells could help. But it is not that simple.
Adult neurogenesis – and atom bombs
Neurons are generally post-mitotic cells, which means that they no longer divide at all. Theoretically, a person dies with the number of neurons he or she was born with (minus a certain amount of cell wear and tear – risky headers in football are currently a topic again). Only the synapses have the potential to constantly re-connect.
Although this view has persisted for a long time, there is now a consensus that neurogenesis takes place in the hippocampus, the area that is crucial for our memory, until old age, meaning that neuronal stem cells continue to differentiate into neurons. To be precise, around 1,400 cells per day, as we know from nuclear weapons tests during the Cold War.
A Swedish research team had examined the brains of people who had died during the Cold War (3). During this time, the atmosphere was enriched with the radioactive isotope C14 due to nuclear weapons tests. This is known from the radiocarbon method used in archaeology, which can be used to calculate the age of a find based on the time it takes to decay. After C14 was emitted in increased quantities by the atomic bombs and absorbed by people, the researchers were able to identify the isotope in the DNA of the hippocampal neurons and, as with an archaeological find, calculate when the respective neurons had their ‘birth date’. The stratification showed that around 700 new cells were generated per hemisphere per day. The good news for patients in neuromedicine is that adult neurogenesis actually takes place every day and is being researched.
Can neural stem cells be used therapeutically?
The bad news: 700 cells per half of the brain is anything but a lot in the human brain with its 80-200 billion neurons, and it certainly won’t compensate for a brain tumor. And since this stem cell miracle is probably limited to the hippocampus, it is of no use in diseases such as Alzheimer’s that affect the entire cortex. Transplantation of such ‘niche stem cells’ has even been attempted on mice – without success. If the stem cells were transplanted outside the hippocampus, the differentiated cells did not work adequately and often died. The idea that we can simply expand or strengthen this neurogenesis is therefore probably doomed to failure.
But there is still a glimmer of hope: things are looking better for fetal neuronal stem cells and the Nobel Prize-winning, laboratory-induced pluripotent stem cells (iPSC), which have been showing success in basic research on mice for some time now. A second article will focus on these successes and the potential for future therapies.
Sources
(1) Kueckelhaus, M., Rothoeft, T., De Rosa, L., Yeni, B., Ohmann, T., Maier, C., … & Hirsch, T. (2021). Transgenic epidermal cultures for junctional epidermolysis bullosa—5-year outcomes. New England Journal of Medicine, 385(24), 2264-2270.
(2) Frangoul, H., Locatelli, F., Sharma, A., Bhatia, M., Mapara, M., Molinari, L., … & Grupp, SA (2024). Exagamglogene Autotemcel for Severe Sickle Cell Disease. New England Journal of Medicine, 390(18), 1649-1662.
(3) Spalding, KL, Bergmann, O., Alkass, K., Bernard, S., Salehpour, M., Huttner, HB, … & Frisén, J. (2013). Dynamics of hippocampal neurogenesis in adult humans. Cell, 153(6), 1219-1227. https://doi.org/10.1016/j.cell.2013.05.002
Figure: Erceg Ivkošić, I., Fureš, R., Ćosić, V., Mikelin, N., Bulić, L., Dobranić, D., … & Primorac, D. (2023). Unlocking the Potential of Mesenchymal Stem Cells in Gynecology: Where Are We Now?. Journal of personalized medicine, 13(8), 1253.

Ethel Purdy – Medical Blogger & Pharmacist
Bridging the world of wellness and science, Ethel Purdy is a professional voice in healthcare with a passion for sharing knowledge. At 36, she stands at the confluence of medical expertise and the written word, holding a pharmacy degree acquired under the rigorous education systems of Germany and Estonia.
Her pursuit of medicine was fueled by a desire to understand the intricacies of human health and to contribute to the community’s understanding of it. Transitioning seamlessly into the realm of blogging, Ethel has found a platform to demystify complex medical concepts for the everyday reader.
Ethel’s commitment to the world of medicine extends beyond her professional life into a personal commitment to health and wellness. Her hobbies reflect this dedication, often involving research on the latest medical advances, participating in wellness communities, and exploring the vast and varied dimensions of health.
Join Ethel as she distills her pharmaceutical knowledge into accessible wisdom, fostering an environment where science meets lifestyle and everyone is invited to learn. Whether you’re looking for insights into the latest health trends or trustworthy medical advice, Ethel’s blog is your gateway to the nexus of healthcare and daily living.



